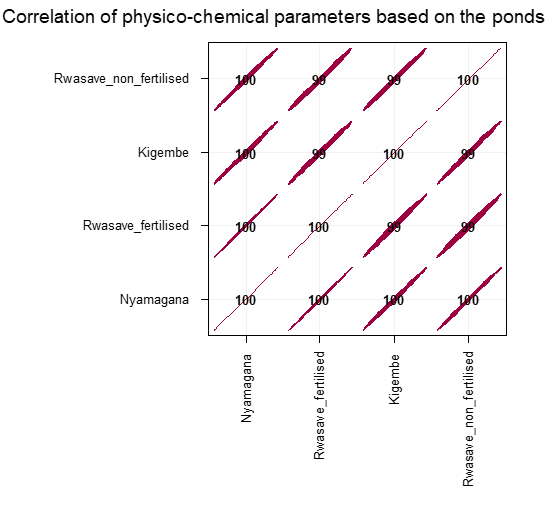J.D. Ndayisenga*
Department of Physics, College of Science and Technology, University of Rwanda, Kigali, Rwanda
S. Dusabe
Department of Chemical Engineering, Institut Technologi Sepuluh Nopember (ITS), Surabaya 60111, Indonesia
*Corresponding author: ndajadhon@gmail.com
Abstract
The anthropogenic activities have caused increase in the aquatic heavy metals pollution. The higher concentration of heavy metals in fish’s body also affects the consumers as it reaches to human body through the food chain. This study was conducted to analyze the ponds’ water quality based on physicochemical parameters and nutrients in relation to the dissolved heavy metals accumulated in the fishes’ body by using R programing. In this study, the heavy metals bio-accumulated from barrage pond and diversions ponds was analysed. Kigembe, Nyamagana, Rwasave fertilized and Rwasave non-fertilized ponds were purposively chosen for the study. The water sample was analysed using HACH DR5000 UV-Vis Spectrophotometer to measure Ammonium-Nitrogen, Nitrate-Nitrogen, Phosphates and Total Phosphorus at Chemistry Department’s Laboratory of University of Rwanda. While after filtration of water samples using Whatman filter papers, heavy metals including Fe, Cu, Mn, Zn, Ni, Co, Cd, Cr and Pb were determined using ICP-MS 7900. The analysis of physicochemical parameters showed that the temperature, pH, Conductivity, Turbidity and TDS were within the permissible limit of ponds’ water (20-300C, 6.5-9, less than 1000 µs/cm, 30-60 NTU and less than 2000 mg/L respectively) quality for all the sites except Rwasave fishponds, where lower pH was observed. The nutrients level in these ponds were low comparing to the standard limit. The assessed heavy metals were Fe, Cu, Mn, Zn, Cd, Cr and Pb whose concentrations analyzed in water were within permissible limit of 0.3ppm, 1ppm, 0.1ppm, 3ppm, 0.003ppm, 0.5ppm and 0.01ppm respectively while heavy metals bio-accumulated were within the permissible limit of 0.1ppm, 1ppm, 0.05ppm, 5ppm, 0.05ppm, 0.05ppm and 0.05ppm respectively for all the sites except for both dissolved and bio-accumulated Fe and Mn concentration which were high for all sites. The highest level of heavy metals concentration was obtained in particular Fe and Mn. This shows that there is urgent need of continuous water quality analysis within the ponds for maintaining the favorite conditions for fish. The water quality monitoring will help the farmers to create the safe aquatic environment for fishes and improve their production output.
Keywords: Heavy metals, Nutrients, Physicochemical parameters, Ponds, Rwanda
DOI: https://doi.org/xx
Conflicts of interest: None
Supporting agencies: None
Received 27.02.2022; Revised 19.04.2022; Accepted 22.04.2022
Cite This Article: Ndayisenga, J.D. & Dusabe, S. (2022). Ponds’ Water Quality Analysis and Impact of Heavy Metals on Fishes’ Body. Journal of Sustainability and Environmental Management, 1(2), X-X. doi: xxxxxxxx
Download full article
1. Introduction
Nowadays, different anthropogenic activities including
agriculture, industries (Joda et al., 2019; Aris et al., 2020) urban run-off
(Adeossun et al., 2015; Montazer et al., 2018) mining activities (Shahbaaet
al., 2020; Soulivongsa et al., 2020) and geochemical structure (Naggar et al.,
2018) increases the aquatic heavy metals pollution (Shahbaaet al., 2020; Ma et
al., 2020). The quality of water affects aquatic life (Olanrewaju et al., 2017;
Tulsankar et al., 2020) this is why ponds’ management is a key during fish
farming (Agyakwah et al., 2020) for its importance for aquatic biodiversity
(Hornbach et al., 2020) as poor water quality reduce fishes yield (Makori et
al., 2017). The existence of trace and accepted heavy metals level are needed
by living body (Shahbaaet al., 2020) including fishes to function and survive
(Shafiuddin et al., 2019) but its higher concentration contaminates the fishes
(mal-growth and reproduction disorder, mainly the skin, gills, liver, spleen,
and kidneys alteration) (Arantes et al., 2015), and even the consumers (human
health) indirectly through food chain (Kamaruzzaman et al., 2020; Liu et al.,
2020). Pb, Cu, Zn are most toxic heavy metals on human body and also affect
environment (Zebib and Teame, 2017; Hadeel et al., 2019; Ma et al., 2020). US
Environmental Protection Agency and even International Agency for research on
cancer classify As, Pb, Cr and Hg as carcinogens (Shahbaaet al., 2020). In
general, heavy metals are higher density (great than 5gmL-1) (Mamboya, 2007)
metallic element, very toxic at even lower concentration (Joda et al., 2019).
Though it is less than 1% mass of living organisms, (Khayatzadeh and Abbasi,
2010) but are not bio-degradable (Abalaka et al., 2020). In this study the
heavy metals bio-accumulated from barrage pond and diversions ponds was
analysed. The dissolved heavy metals reach directly to the fish tissues
(Khayatzadeh and Abbasi, 2010; Adebayo, 2017; Rajeshkumar and Li, 2018; Liu et
al., 2020) by gills, body surface and also digestive track (Afshan et al.,
2014), which is excreted via the feces, urine, and respiratory membranes (Joda
et al., 2019). The concentration of heavy metals is not balanced in all organs
of the fishes (Jia et al., 2017; Rajeshkumar and Li, 2018; Tulsankar et al.,
2020). According to Amal and Nahed (2012), the higher concentration was
observed in intestine than muscle. The highest concentration was observed in
the liver tissues of fish, while the least concentration was observed in bone
tissues (Uwem et al., 2013). The fish’s composition varies due to different
factors even on the same species, with major factors including seasonal
variation, environment, sexual cycle, maturity stage, feed, organs and also
muscle location (Talab et al., 2016). The aquatic heavy metals concentration affects
the fish size, with the linkage to the ecological needs, swimming behaviours
and also metabolic activity (Yia and Zhang, 2012; Zebib and Teame, 2017),
growth and increase fishes’ developmental anomalies (Khayatzadeh and Abbasi,
2010). Even Jia et al., (2017) found that it is at low probability p<0.01. Physio-chemicals
parameters (physical, chemical and biological characteristics) (Zebib and
Teame, 2017) indicate the nature and quality of water contained in the ponds
(Mohamed, 2005; Ma et al., 2020) as the basic factor to control the dynamics
and even structure of aquatic life (Makori et al., 2017; Ndayisenga and
Habimana, 2020) and affect type and amount of nutrients (Chen et al., 2018).
The presence of mineral concentrations in fish muscle affect different
biological factors (Talab et al., 2016) while the presence of higher nutrients
level causes the eutrophication of water (Chen et al., 2018) and becomes threat
to the fish life (Kane et al., 2015). A study has shown that the nutrient level
and organic matter is high in the ponds than in the rivers (Dróżdż et al.,
2019). The water temperature, dissolved oxygen and saturated dissolved oxygen are
the major parameters affecting fish distribution (Yağcı et al., 2015;
Ndayisenga and Habimana, 2020).
2. Materials and methods
2.1. Sampling
location
The three spatial distributed sampling sites were chosen,
based on its highest production compared to the other ponds and the long period
of serving the community. Since 1954, Kigembe pond was established between Gisagara
and Nyaruguru Districts in south of Rwanda for small scale fish farming. The
inlet water in this ponds is from the Migina River. Nyamagana fish pond is
located in Nyanza district, Southern province of Rwanda about 2 km from Nyanza
town. Rwasave fish farming is in Huye district (the study consider fertilized
and non-fertilized ponds) and the inlet water comes from Rwabuye River. The
types of fish produced at the stations are Tilapia
nilotica and Clarias gariepinus.
2.2. Sample
preparation and analysis
Some parameters including pH, TDS, conductivity and
temperature were measured at the field using multiparameter and followed by
taking the water samples using a well rinsed and acid-cleaned polyethylene
bottles for heavy metals and nutrients analysis. The samples were transported
to the University of Rwanda, Chemistry Department’s Laboratory for analysis.
Using HACH DR5000 UV-Vis Spectrophotometer, nutrients including
Ammonium-Nitrogen (NH4-N), Nitrate-Nitrogen (NO3-N),
Phosphates (PO43-) and Total Phosphorus (TP) were
measured. While after filtration of water samples using whatman filter papers
(Cat No.1001 150), heavy metals including Fe, Cu, ¬Mn, Zn, Ni, Co, Cd, Cr and
Pb were determined using ICP-MS 7900. For heavy metals analysis in fish
samples, the fishes’ intestine was removed first and the remaining part were
dried in oven at 700C for 48 hours, followed by crushing together with pestle
and mortar into fine powder. 1.250g of this powder was put in 125mL digestion
flasks and digested in concentrated nitric acid (69% HNO3, ANALAR
Grade) and 30% H2O2 by heating and cooling processes from
1000C to 2000C. The solutions were evaporated to 5 ml
until no brown fumes evolved for about 3 hours. After cooling, the solutions
were kept into volumetric flasks of 250 ml and filled up to the mark using
distilled water. The sample solutions were transferred in Teflon bottles and
settled over 15 hours. The digested sample solutions were filtered through
Whatman filter papers (Cat No.1001 150) into volumetric flasks and heavy metals
were measured as done on water sample.
3. Results and discussion
3.1. Physicochemical parameters
The aquatic temperature influence generally aquatic life and in particular the metabolism rate (Ndayisenga and Habimana, 2020). Temperature is proportional to the solubility of solute contained in water, the rate of reaction, rate of bio-chemical activity of the micro biota, plant respiratory rate, evaporation, and vaporization of the water content. For all the sampled ponds, based on guideline for aquatic life (Bhavimani and Puttaiah et al., 2014), the temperature was in acceptable range for fish life (Figure 1 and Table 3), thus the fishes are not stressed by temperature in the ponds. Some factors including season, diurnal sampling time, depth, cloud cover, air circulation and flow affect the magnitude of the water’s temperature. Generally, there is negative correlation of physicochemical parameters along the ponds as mentioned on corplot (Figure 3).
Table 1: Heavy metals concentration levels in water sample
|
Heavy metals |
Kigembe pond |
Nyamagana pond |
Rwasave fertilized |
Rwasave non fertilized |
|
Chromium |
0.00094 |
0.00005 |
0.00049 |
0.00031 |
|
Manganese |
0.145 |
0.144 |
0.17 |
0.18 |
|
Iron |
1.849 |
0.235 |
0.779 |
1.87 |
|
Cobalt |
0.00098 |
0.0009 |
0.00096 |
0.00086 |
|
Nickel |
0.00347 |
0.003 |
0.0023 |
0.0032 |
|
Copper |
0.0045 |
0.002 |
0.0025 |
0.0038 |
|
Zinc |
0.078 |
0.025 |
0.0097 |
0.027 |
|
Cadmium |
0.00063 |
0.00019 |
0.00092 |
0.00039 |
|
Lead |
0.0026 |
0.00145 |
0.0093 |
0.0016 |
Table 2: Heavy metals concentration levels in sampled fishes
|
Heavy metals |
Kigembe pond |
Nyamagana pond |
Rwasave fertilized |
Rwasave non fertilized |
|
Chromium |
0.025 |
0.032 |
0.026 |
0.028 |
|
Manganese |
0.174 |
0.186 |
0.069 |
0.19 |
|
Iron |
0.96 |
1.3 |
0.61 |
1.39 |
|
Cobalt |
0.00083 |
0.0015 |
0.001 |
0.011 |
|
Nickel |
0.014 |
0.019 |
0.013 |
0.025 |
|
Copper |
0.022 |
0.025 |
0.014 |
0.023 |
|
Zinc |
0.18 |
0.39 |
0.27 |
0.6 |
|
Cadmium |
0.011 |
0.0011 |
0.006 |
0.00063 |
|
Lead |
0.0065 |
0.0069 |
0.17 |
0.014 |
Table 3: Physico-chemical and nutrients concentrations levels in the
water body
|
Parameters |
Kigembe pond |
Nyamagana pond |
Rwasave fertilized |
Rwasave non fertilized |
|
Temperature (oC) |
23.9 |
27.1 |
24.8 |
26.3 |
|
PH |
8.72 |
8.01 |
6.32 |
5.84 |
|
E.C (µS/cm) |
96.1 |
113.5 |
112.2 |
86.8 |
|
Turbidity (NTU) |
53.7 |
56 |
50 |
48 |
|
TDS (mg/L) |
54.2 |
53.8 |
53.2 |
40.7 |
|
Ammonia-Nitrogen (mg/L) |
0.2 |
0.22 |
0.1 |
0.1 |
|
Nitrate-Nitrogen (mg/L) |
0.1 |
0.3 |
0.2 |
0.1 |
|
Total Nitrogen (mg/L) |
0.333 |
0.57 |
0.33 |
0.29 |
|
Phosphate (mg/L) |
0.18 |
0.05 |
0.15 |
0.13 |
|
Total Phosphorus (mg/L) |
0.33 |
0.33 |
0.42 |
0.17 |
pH
The acidity and basicity of the ponds’ water influences
biological and chemical processes within a water body (Mohamed, 2005). The high
pH present in Kigembe fishpond was attributed to the type of soil containing Ca2CO3
and MgCO3 in its structure therefore playing a key role in this high
pH values recorded. The low pH present in Rwasave ponds (Figure 1 and Table 3)
are also attributed to the rabbit farming above the pond and pigs, chickens surrounding
those ponds. The CO2 dissolved in water form H2CO3
which decrease the pH value and becomes harmful to the aquatic life (Hemalatha
and Puttaiah, 2014) by increasing stress levels and causing slow growth, this
is why there is a need to regularize the ponds’ pH.
Electrical
Conductivity
The electrical conductivity values in water samples were
below the permitted value (<1000 µS/cm) recommended by Rwanda Standard Board
guideline (Figure 1 and Table 3). This is the ability of water to conduct
current and it is the results of presence of charged particles (Ndayisenga and
Habimana, 2020). The water conductivity is proportional to the dissolved salts
and also increases with temperature.
Total
dissolved solids (TDS)
The ponds’ water body dissolved solids include inorganic
salts like calcium, magnesium, potassium, sodium, bicarbonates, chlorides and
sulphates and even few organic materials originating from leaves, silt,
plankton and sometimes industrial waste and sewage. This parameter indicates
hardness of the water. For all sampled ponds TDS were below the standard value
of surface water (Figure 1 and Table 3).
Turbidity
The ponds’ suspended matters including grains, grey,
planktons and organic matters are the major cause of highest light scattering
than the reference (Hemalatha and Puttaiah, 2014). This parameter varies
seasonally as aquatic biological activities and surface run-off varies. Diurnal
turbidity variation may take place depending mostly on rainfall (Ndayisenga and
Habimana, 2020). In case of brownish ponds’ water, it indicates the presence of
clay while greenish indicate presence of plankton. Even the sample was taken
during rainy season it didn’t cause to exceed maximum permissible limit (Figure
1 and Table 3).
3.2. Nutrients
Total
nitrogen
This is
determined by the nitrogen in its different forms including NH3, NO3-, NO2- and
organic nitrogen. The main total Nitrogen concentration comes from the
decomposition of human wastes, plant decomposition, livestock wastes and runoff
from fertilizers for agricultural purpose and the discharge of municipal wastes
into ponds. The amount of municipal waste is in increasing trend due to
increase in population (Giri, 2021; Khanal, Sondhi and Giri, 2021) causing threat
to the aquatic ecosystem. The fishponds feeding with organic manure increase
the nutrients in fishponds for maximizing production and this must be
controlled to limit excess nutrient feed. The lower concentration of TN was
observed at Rwasave non fertilized pond (Figure 1 and Table 3), this means that
the practice of fertilizing fishponds is needed.
Ammonium
ions
NH4+
is a nitrogen source and used by algae and plants. Than nitrate, the NH3
is toxic while NH4+ is not but the two forms are grouped together as
total ammonia. The values of ammonium ion concentrations were 0.2, 0.22, 0.1
and 0.1 at Kigembe, Nyamagana, Rwasave fertilized and Rwasave non-fertilized
ponds respectively (Figure 1 and Table 3). Thus, ammonium ion levels are
normally stabilized in the 0-2 mg/l as desirable range.
Total
phosphorus and phosphates
This is a key
nutrient for stimulating aquatic plants and algae growth, resulting in the
eutrophication of water bodies. The phosphate levels are normally stabilized in
the range of 0.01-3 mg/l. This study showed that phosphate concentrations (in
mg/l) were 0.18, 0.05, 0.15 and 0.13 at Kigembe, Nyamagana, Rwasave fertilized
and Rwasave non-fertilized ponds respectively while the total phosphorus (TP)
were 0.33, 0.33, and 0.42, 0.17 mg/l respectively (Table 3). Rwanda standard
board guideline for TP levels are normally stabilized in the < 3 mg l-1
as desirable range for surface water. The high phosphate and TP observed at
Kigembe and Rwasave fertilized ponds were attributed to rabbits, pigs and
chickens manure droppings in the ponds. Thus, fertilizers from marshland of
Rwabuye in the rice plantation increase the level of TP and Phosphate
concentration of Rwasave ponds.
3.3. Heavy metals
The finding of
this research on heavy metals are summarized in Figure 2 and Figure 3. Figure 3
relate the concentration in fishes’ tissues versus that of water, and these
figures shows that heavy metals are concentrated in fishes’ body than in water.
But some heavy metals concentration is high in water than in fishes for very
few sampling station like Mn at Rwasave fertilized, Fe at Kigembe, Rwasave
fertilized and Rwasave non fertilized, Co at Kigembe pond (Table 1 and Table
2).
Manganese
(Mn)
Mn is among the
most abundant metals element in Earth’s crust. It supports animals functioning
through its cellular enzymes including manganese superoxide dismutase. For
water samples, the Mn concentrations (in ppm) were 0.145, 0.144, 0.17 and 0.18
at Kigembe, Nyamagana, Rwasave fertilized and Rwasave non-fertilized ponds respectively
(Table 1) while for Mn accumulated by fishes (in ppm) were 0.174, 0.186, 0.069
and 0.19 at Kigembe, Nyamagana, Rwasave fertilized and Rwasave non-fertilized
ponds respectively (Table 2). These results are above the recommended values of
0.1ppm for ponds water and 0.05 ppm for fishes. Therefore, this indicates that
water and fishes from the studied ponds are polluted with Mn. This pollution
was attributed to weathering of soils and rocks that are habitually discharged
in Kigembe, Nyamagana, Rwasave fertilized and Rwasave non-fertilized ponds.
Chromium
(Cr)
Cr is naturally
found in rocks and soil and is very persistent in water sediments. The metal is
used in metal alloys and pigments for paints, cement, paper, rubber and other
materials. Its chronic exposure to human cause kidney, liver damage,
circulatory and nerve tissues. For water samples, the Cr concentrations (in
ppm) were 0.000094, 0.00005, 0.00049 and 0.00031 at Kigembe, Nyamagana, Rwasave
fertilized and Rwasave non-fertilized ponds respectively (Table 1 and Figure 2)
while for fishes, its concentrations in ppm were 0.025, 0.032, 0.026 and 0.028
at Kigembe, Nyamagana, Rwasave fertilized and Rwasave non-fertilized ponds
respectively (Table 2).
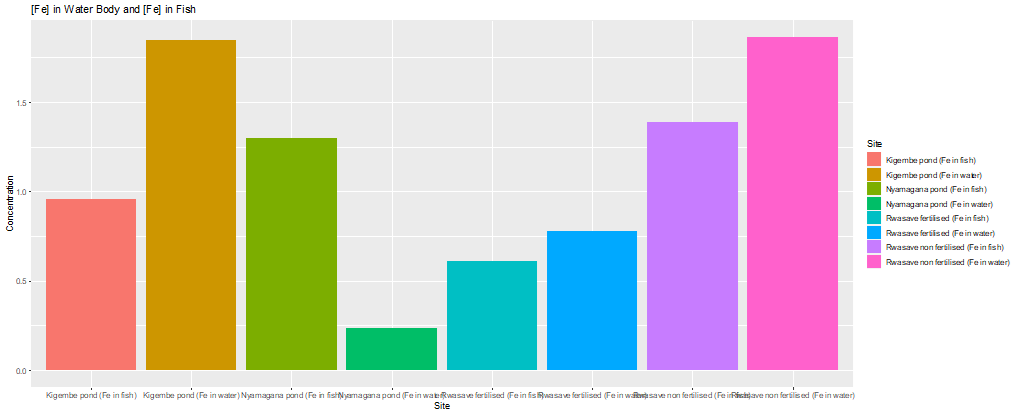
Figure 2: The comparison plot of measured heavy metals concentration in ponds’ water versus that in fishes’ tissues
Figure 3: The comparison plot of measured heavy metals concentration in ponds’ water versus that in fishes’ tissue
These results are below the recommended values of 0.50ppm for ponds water and 0.05 ppm for bio accumulated concentration. These low concentrations of Cr were due to less agro-chemical usage around the study areas and less industrial activities which are the major sources of Cr aquatic pollution.
Lead (Pb)
Pb is among the
heavy metals with specific toxicity and cumulative effects. The main aquatic
sources are lead processing industries (Ndayisenga and Habimana, 2020). Some of
its health effects include liver damage, kidney and reduction in haemoglobin
formation, mental retardation, infertility and abnormalities in case of
pregnancy, and at strong contaminated it cause gastrointestinal disorders,
constipation, abdominal pain, neuromuscular effects weakness, nervous system
effects or syndrome that may result to coma or death. For water samples, the Pb
in ppm were 0.0026, 0.00145, 0.00093 and 0.0016 at Kigembe, Nyamagana, Rwasave
fertilized and Rwasave non-fertilized ponds respectively (Table 1 and Table 2)
while for fishes were 0.0065, 0.0069, 0.17 and 0.014 at Kigembe, Nyamagana,
Rwasave fertilized and Rwasave non-fertilized ponds respectively (Table 2).
These results are below the recommended values of 0.01 ppm for ponds water and
0.05 ppm for bio accumulated in fishes. Thus, the water from the studied ponds
are not polluted with Pb, due to less anthropogenic sources like industrial and
municipal wastewater discharges, mining around study areas which are the among
the major sources of aquatic Pb concentration.
Iron (Fe)
Fe is involved
in the haemoglobin synthesis in the red blood and is a necessary element in
human diet and has a significant role in metabolic processes, in case of too
little Fe in the body; the iron deficiency (anemia) was developed (Arantes et
al., 2015). For water samples, the Fe concentrations (in ppm) were 1.849,
0.235, 0.779 and 1.87 (Table 1 and Figure 2) while for fishes were 0.96, 1.30,
0.61 and 1.39 at Kigembe, Nyamagana, Rwasave fertilized and Rwasave
non-fertilized ponds respectively (see table 2 and figure 2). Simply, the
results are above the recommended values of 0.3ppm for ponds water except at
Nyamagana and 0.1ppm for fishes. Therefore, the ponds’ water and fishes are
polluted with Fe. This high concentration of Fe should be raised by run-off
rusting materials and sewage effluents containing iron into these ponds.
Naturally, rocks and soil weathering should increase the level of iron
concentration at studied ponds.
Cupper (Cu)
Cu is a
low-toxicity, corrosion-resistant metal widely used because of its ductility
and malleability, electrical conductivity, and ability to conduct heat. Cu is
also used in tubing and piping. For water samples, Cu concentrations (in ppm)
were 0.0045, 0.002, 0.0025 and 0.0038 (Table 1 and Figure 2) while for fishes
were 0.022, 0.025, 0.014 and 0.023 at Kigembe, Nyamagana, Rwasave fertilized
and Rwasave non-fertilized ponds respectively (Figure 2 and Table 2). These
results are below the recommended values of 1.0ppm for ponds water and 1.0 ppm
for fishes. This indicates that water and fishes from the studied ponds are not
polluted by Cu.
Zinc (Zn)
Earth's crust is
one of the main sources of Zn. This is an enzyme co-factor in several enzyme
systems including carbonic anhydrase found in red blood cells. Like other
metals it is emitted from its natural and anthropogenic sources (Arantes et
al., 2015). For water samples, the Zn concentrations (in ppm) were 0.078,
0.025, 0.0097 and 0.027 (see table 1 and figure 2) while for fishes were 0.18,
0.39, 0.27 and 0.60 at Kigembe, Nyamagana, Rwasave fertilized and Rwasave
non-fertilized ponds respectively (Figure 2 and Table 2) which are under the
recommended values of 3.0ppm for ponds water and 5.0 ppm for fishes. This
indicates that water and fishes are not polluted by Zn. Thus, Zn concentrations in fishes were
greater than in water; this confirms that sediments are repository of metals
and indicated a certain degree of bio-accumulation.
Cadmium (Cd)
During metals
plating Cd is used and is toxic at even low concentrations, non-biodegradable,
non-essential heavy metals and have no role in biological processes in living
tissues. Thus, even in low concentration, it could be harmful to fish. For
water samples, the Cd (in ppm) were 0.00063, 0.00019, 0.00092 and 0.00039
(Table 1 and Figure 2) while for fishes were 0.011, 0.0011, 0.006 and 0.00063
ppm at Kigembe, Nyamagana, Rwasave fertilized and Rwasave non-fertilized ponds
respectively (see table 2 and figure 2). These results are below the
recommended values of 0.003ppm for ponds water and 0.05 ppm for fishes. Cd
concentrations in fishes were also greater than in water, this difference in
the pattern of heavy metals in these fish samples might be a result of their
difference in many factors such as feeding habits, habitats, ecological needs,
metabolism and biology.
4. Conclusion
Based on the
study finding, the water quality based on physicochemical parameters, nutrients
analysis and even heavy metals concentration have great health effect to the
fishes’ community. The physicochemical and nutrients parameters for water body
mainly influence the abundance and fertility of fishes, and are tolerant at a
certain level. The heavy metals are bio accumulated in fishes, which means the
more heavy metals content in water body means more bio accumulation. Therefore,
during implementation of fishes farming projects, the ponds’ water quality must
be assessed periodically. The water quality monitoring will help the farmers to
create the safe aquatic environment for fishes and improve their production
output. This will reduce the health effects related to the consumption of
contaminated fishes as the contamination level depends on species and different
aquatic environment. Food chain becomes the main route of accumulating toxic
contained in fishes by human body.
Acknowledgements
The authors
would like to thank the University of Rwanda, College of Science and
Technology, Chemistry Department for giving access to the reagents and
instruments used during sample analysis.
References
Adebayo, I. A. (2017).
Determination of heavy metals in water, fish and sediment from Ureje water reservoir.
Journal of Environmental & Analytical
Toxicology, 7(4), 1-4.
Adeossun, F. I., Akinyemi,
A. A., Idowu, A. A., Taiwo, I. O., Omoike, A., & Ayorinde, B. J. O. (2015).
The effect of heavy metals concentration on some commercial fish in Ogun river,
Opeji, Ogun State, Nigeria. African
Journal of Environmental Science and Technology, 9(4), 365-370.
Afshan, S., Ali, S., Ameen,
U. S., Farid, M., Bharwana, S. A., Hannan, F., & Ahmad, R. (2014). Effect
of different heavy metal pollution on fish. Research
Journal of Chemical and Environmental Sciences, 2(2), 34-40.
Agyakwah, S. K., Asmah, R.,
Mensah, E. T. D., Ragasa, C., Amewu, S., Tran, N., Oyih, M. & Ziddah, P.
(2020). Farmers’ manual on small-scale
tilapia pond farming in Ghana. CSIR- Water Research Institute, Accra,
Ghana, Tech. Rep. TR- CSIR/WRI/MA/SKA/2020/2.
Amal, M. Y., & Nahed,
S. G. (2012). Accumulation of some heavy metals and biochemical alterations in
muscles of Oreochromis niloticus from the River Nile in Upper Egypt. International Journal of Environmental
Science and Engineering, 3(13), 1-10.
Arantes, F. P., Savassi, L.
A., Santos, H. B., Gomes, M. V. T., & Bazzoli, N. (2015). Bioaccumulation
of mercury, cadmium, zinc, chromium, and lead in muscle, liver, and spleen
tissues of a large commercially valuable catfish species from Brazil. Anais da Academia Brasileira de Ciências,
88(1), 137-147.
Aris, M., & Tamrin.
(2020). Heavy metal (Ni, Fe) concentration in water and histopathological of
marine fish in the Obi Island, Indonesia. Jurnal
Ilmiah Platax, 8(2), 221-233.
Bhavimani, H., &
Puttaiah, E. T. (2014). Fish culture and physico-chemical characteristics of
madikoppa pond, Dharwad Tq/Dist, Karnatak. Hydrology
Current Research, 5(1), 1-3.
Chen, R., Ju, M., Chu, C.,
Jing, W., & Wang, Y. (2018). Identification and quantification of
physicochemical parameters influencing chlorophyll-a concentrations through
combined principal component analysis and factor analysis: A case study of the
Yuqiao reservoir in China. Sustainability
MDPI, 10(936), 1-15.
Chikaire, J.U., Ajaero,
J.O., & Atoma, C.N. (2022). Socio-economic effects of covid-19 pandemic on
rural farm families’ well-being and food systems in Imo State, Nigeria. Journal of Sustainability and Environmental
Management, 1(1), 18-21.
Dróżdż, D., Malińska, K.,
Mazurkiewicz, J., Kacprzak, M., Mrowiec, M., Szczypiór, A., Postawa, P., &
Stachowiak, T. (2019). Fish pond sediment from aquaculture production – current
practices and the potential for nutrient recovery: A Review. International Agrophysics, 34(5), 33-41.
Giri, S. (2021). Integrate
solid waste management: A case study of a hotel in Kathmandu, Nepal. EPRA International Journal of
Multidisciplinary Research, 7(5), 264-268.
Hadeel, M. H., & Ahmed,
M. J. (2019). Heavy metals causing toxicity in fishes. 2nd International Science Conference in IOP Conf. Series: Journal of
Physics: Conf. Series 1294 (2019) 062028, Baghdad, Iraq, 1–10.
Hemalatha, B., &
Puttaiah, E. T. (2014). Fish culture and physico-chemical characteristics of
madikoppa pond. Hydrology Current Research,
5(1), 1-3, doi: 10.4172/2157-7587.1000162.
Hornbach, D. J., Schilling,
E.G., & Kundel, H. (2020). Ecosystem metabolism in small ponds: The effects
of floating-leaved Macrophytes. Water
MDPI., 12(1458), 1-25.
Jia, Y., Wang1, L., Qu, Z.,
Wang, C., & Yang, Z. (2017). Effects on heavy metal accumulation in
freshwater fishes: species, tissues, and sizes. Environment Science Pollution Research, 24(3), 9379-9386.
Joda, B. A., Alheloo, H.
S., Al-Mankosh, H. J. A., & Maitham, S.A. (2019). Determination of heavy metals
arsenic, cadmium and lead in water, sediments and fish from Al Delmaj
Marshes-Iraq. The 7th International Conference on Applied Science and Technology
(ICAST 2019) AIP Conference Proceedings 2144,
2019, 020012-1–020012-8.
Kamaruzzaman, Y., Zuraidah,
M. A., & Akbar, J. (2020). A review on the accumulation of heavy metals in
coastal sediment of Peninsular Malaysia. Ecofeminism
and Climate Change, 1(1), 21-35.
Kane, S., Qarri, F., Lazo, P.,
& Bekteshi, L. (2015). The effect of physico-chemical parameters and nutrients
on fish growth in Narta Lagoon, Albania. Journal
of Hygienic Engineering and Design, 62-68.
Khanal, A., Sondhi, A.,
& Giri, S. (2021). Use of personal protective equipment among waste workers
of Sisdol landfill site of Nepal. International
Journal of Occupational Safety and Health, 11(3), 158-164.
Khayatzadeh, J., &
Abbasi, E. (2010). The effects of heavy
metals on aquatic animals. The 1st International Applied
Geological Congress, Department of Geology, Islamic Azad University - Mashad
Branch, Iran, 688-694.
Liu, Q., Liao, Y., Xu, X.,
Shi, X., Zeng, J., Chen, Q., & Shou, L. (2020). Heavy metal concentrations
in tissues of marine fish and crab collected from the middle coast of Zhejiang
Province, China. Environment Monitoring
Assessment, 192(285), 1-12.
Ma, J., Wu, S., Shekhar, N.
V. R., Biswas, S., & Sahu, A. K. (2020). Determination of physicochemical
parameters and levels of heavy metals in food waste water with environmental effects.
Bioinorganic Chemistry and Applications,
1-9.
Makori, A. J., Abuom, P.
O., Kapiyo, R., Anyona, D. N., & Dida, G. O. (2017). Effects of water
physico-chemical parameters on tilapia (Oreochromis niloticus) growth in
earthen ponds in Teso North Sub-County, Busia County. Fisheries and Aquatic Sciences, 20(30), 1-10.
Mamboya, F. A. (2007). Heavy metal contamination and toxicity,
Studies of Macroalgae from the Tanzanian Coast. Stockholm University,
Sweden.
Mohamed, H. A. (2005).
Physico-chemical characteristics of abu za'baal ponds, Egypt. Egyptian Journal of Aquatic Research, 31(2),
1-15.
Montazer, M., & Ali, S.
(2018). Determination of heavy metals in freshwater fishes of the Tigris River
in Baghdad. Fishes MDPI, 3(23), 1-6.
Naggar, Y. A., Khalil, M.
S., & Ghorab, M. A. (2018). Environmental pollution by heavy metals in the
aquatic ecosystems of Egypt. Open Access
Journal of Toxicology, 3(1), 001-009.
Ndayisenga, J. D., &
Habimana, V. (2020). The use of aquatic macro-invertebrate and physico-chemical
parameters for water quality analysis in wetlands of Kigali city. East African Journal of Science and
Technology, 10(3), 91-104.
Olanrewaju, A. N., Ajani,
E. K., Kareem, O. K., & Orisasona, O. (2017). Relationship between
physico-chemical parameters and reproductive indices of parachanna obscura
(Gunther 1861) in eleyele reservoir, Ibadan, Nigeria. European Journal of Experimental Biology, 7(6:36), 1-6.
Rajeshkumar, S., & Li,
X. (2018). Bioaccumulation of heavy metals in fish species from the Meiliang
Bay, Taihu Lake, China. Toxicology
Reports, 5, 288-295.
Shafiuddin, A. S. A.,
Sharmin, S., Ahasan, H., Hadayet, U., Najiah, M., Mahfujur, M. R., &
Shafiqul, M.I.S. (2019). Bioaccumulation
of heavy metals in commercially important fish species from the tropical river
estuary suggests higher potential child health risk than adults.
Chittagong, Bangladesh.
Shahbaa, K. A., Karam, H.,
& Hana, K. I. (2020). Review on some heavy metals toxicity on freshwater fishes.
Journal of Applied Veterinary Sciences,
5(3), 78-86.
Soulivongsa, L.,
Tengjaroenkul, B., & Neeratanaphan, L. (2020). Effects of contamination by
heavy metals and metalloids on chromosomes, serum biochemistry and
histopathology of the bonylip barb fish near sepon gold-copper mine, Lao PDR. International Journal of Environmental
Research and Public Health, 17(9492), 1-16.
Talab, A. S., Goher, M. E.,
Ghannam, H. E., & Abdo, M. H.
(2016). Chemical compositions and heavy metal contents of Oreochromis
niloticus from the main irrigated canals (rayahs) of Nile Delta. Egyptian Journal of Aquatic Research, 42(3),
23-31.
Tulsankar, S. S., Cole, A.
J., Gagnon, M. M., & Fotedar, R. (2020). Temporal variations and pond age
effect on plankton communities in semi-intensive freshwater marron (Cherax
cainii, Austin and Ryan, 2002) earthen aquaculture ponds in Western Australia. Saudi Journal of Biological Sciences,
28, 1392-1400.
Uwem, G. U., Asuquo, F. E.,
Idung, J. U., & Andem, B. (2013). Bioaccumulation of heavy metal in three
fresh water fishes caught from cross river system. European Journal of Experimental Biology, 3(3), 576-582.
Yağcı, A., Yağcı, A. M.,
Bilgin, F., & Erbatur, I. (2015). The effects of physicochemical parameters
on fish distribution in Eğirdir Lake, Turkey. Iranian Journal of Fisheries Sciences, 15(2), 846-857.
Yia, Y. J., & Zhang, Z.
H. (2012). The relationships between fish heavy metal concentrations and fish
size in the upper and middle reach of Yangtze River. Procedia Environmental Sciences, 13(1), 1699-1707, 2012.
Zebib, H., & Teame, T. (2017). Assessment of monthly physico-chemical properties and fish yields of two micro dams of Tigray Region, Northern Ethiopia. International Journal of Fisheries and Aquaculture, 9(9), 92-97.
|
|
©
The Author(s)
2022. This article is an open access article distributed under the terms and conditions
of the Creative Commons Attribution (CC BY) license. |