Khalefa A. Faneer*
Department of Environment Engineering, Higher Institute of Science and Technology, Bent Baya, Wadi AL-Ajal, Libya
Ebrahim Mahmoudi
Department of Chemical & Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, UKM, Bangi, Malaysia
Muneer Ba‑Abbad
Department of Chemical Engineering, Faculty of Engineering and Petroleum, Hadhramout University of Science &Technology, Mukalla, Hadhramout, Yemen
Rosiah Rohani
Department of Chemical & Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, UKM, Bangi, Malaysia
*Corresponding author: k.fneer@niclibya.com
Abstract
The urgent concern of water shortage have promoted to the development of different sustainable technologies with the rapid evolution of nanotechnologies. Graphene oxide (GO) is a water-soluble material that can be constructed into a number of structures such as membranes, and has many applications in environmental sectors. Furthermore enhancing membrane characteristics to improve water flux performance and mitigate fouling is crucial issue for membrane separation technology. GO serves as nanofiller to overcome fouling during filtration as well as water flux improvement. This work aimed to develop PES membranes by phase inversion method and the fabricated membranes subjected to series analysis of FESEM, hydrophilicity and water flux. The results showed that the GO improved the contact angle of the pure PES membrane and the water flux increased from 13 to 16 L/m2.h. Therefore, the PES-GO membrane proved its capability to be used for various applications to reduce membrane fouling.
Keywords: Fouling, Graphene oxide, Membrane, Nanocomposite, PES, Water
DOI: https://doi.org/x
Received 28.02.2022; Revised 02.05.2022; Accepted 07.05.2022
Cite This Article: Faneer, K.A., Mahmoudi, E., Ba‑Abbad, M., & Rohani, R. (2022). Graphene Oxide Nanocomposite for Sustainable Pure Water by PES Membrane. Journal of Sustainability and Environmental Management, 1(2), x-x. doi: xxxxxxxx
Download full article
1. Introduction
Water resource sustainability is essential for the
long-term evolution of modern society and economy. More strain on water
resources deficit has been developed as a result of industrialization and human
activities, which has accompanied the rapid expansion of the economy and
society (Al Aani, Mustafa, & Hilal, 2020). Due to high growing in industry,
the oily wastewater has increased dramatically, thus the surrounding
environment such as drinking water as well as ground water and sea water being
impacted significantly. As a result, the harm to the ecosystem as a whole may
increase. Membranes, electrochemical, biological, UV irradiation, hybrid
technologies, and destabilization of emulsions by adding minerals are only a
few of the approaches that have been tested so far to remove oil from water. A
variety of membranes, including those generated through interfacial
polymerization, nanoparticle incorporation, and surface grafting, have been
emphasized. (Bolto, Zhang, Wu, & Xie, 2020). Membrane technology, particularly
Ultrafiltration (UF) membranes, has advanced significantly since the 1970s as a
safe, clean, cost-effective, and effective separation method for a variety of
components and contaminants in water and wastewater (Ismail, Khulbe, &
Matsuura, 2015). Due to uniformity in the separation process, no chemicals
added in or during the filtration process, high separation performance
efficiency, ease of operation, and small footprint, polymeric membranes play a
large role in separating oil from oil-water emulsions. The membrane acts as a
barrier, preventing oil droplets from flowing through but allowing water
molecules with smaller hydrodynamic particle sizes to pass through. In general,
the membrane's surface parameters (pore size, porosity, surface wettability, and
surface roughness) have a big impact on both permeate flux and rejection (Teow
& Solihuddin, 2020).
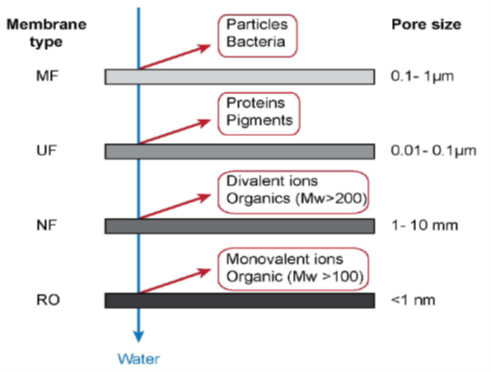
Microfiltration (MF), ultrafiltration (UF),
nanofiltration (NF), reverse osmosis (RO), forward osmosis (FO), and membrane
distillation (MD) are examples of novel water purification and reclamation
techniques (Zhang et al., 2016). These membranes are classified based on pore
size measurements (Figure 1), furthermore, each filtration process works in the
specific range of operating pressure (Kumar et al., 2013). Among these
membranes, MF and UF are the major filtration processes used for oil removal.
Furthermore, hydrophilic surfaces will repulse oil droplets from the membrane
and facilitate the passage of water (Zhu, Loo, & Bai, 2013). Membrane
fouling has been a barrier in membrane water treatment technology since its
inception, reducing water permeation flux, degrading product water quality, and
increasing energy usage (Shannon et al., 2010). Membrane fouling can result in
a temporary or permanent decrease in flux as a result of pore clogging, cake
formation, organic and inorganic precipitation, and biological fouling. Because
of direct interaction with various types of foulants in the raw water,
practically all membrane processes suffer from fouling difficulties when used
for long-term water purification (Zhang et al., 2016).
In order to deal with membrane fouling, various routs have been attempted, such as: surface modification by incorporating antifouling materials. In recent years, the Graphene (G) and Graphene Oxide (GO) have become one of the most important commercial nano-sheets in the world. GO is the derivative of the graphene and it has been extensively explored for the adsorption of various pollutants (Baig, Sajid, & Saleh, 2019). Researchers concern attracted to graphene (G) and graphene oxide (GO) due to many reports on their mechanical and chemical properties when incorporated with the membranes ( Yeh, Cihlář, Chang, Cheng, & Teng, 2013; Mahmoudi, Ng, Ba-Abbad, & Mohammad, 2015). The GO materials have a great potential for absorbing organic molecules in aqueous solutions. (G. P. Rao, Lu, & Su, 2007), the GO membrane is extremely effective in removing organic pollutants and dyes from solution. Adsorption, flotation, and biological treatment are some of the technologies used in industry to treat produced water for disposal or reuse in other processes. These technologies, on the other hand, are ineffective for de-oiling water with droplet sizes smaller than 10 μm. Hybrid techniques have also been tried to break down the oil droplets, but these are energy-intensive and environmentally damaging. Membrane technology, on the other hand, has been recognized as a viable approach for oil emulsion separation that uses less energy and has a reduced environmental impact. (Alammar, Park, Williams, Derby, & Szekely, 2020). Rao et al. (2014) reported efficient removal of 4-chlorophene, 2,4-dichlorophene, and 2,4,6-trichlorophene by ZrO2 graphene composite compared pure membranes. Chung, Y.T., et al. stated that the PSf composite membrane with Zn-GO exhibits excellent antifouling and antibacterial properties (Chung et al., 2017). Furthermore, the advantages of using GO as a nanoplates with nanoparticles is to offer a good and homogenous distribution of the nanoparticles across the membrane matrix (Mahmoudi et al., 2015). Various kinds of polymer including Polyethersulfone (PES), Polysulfone (PSf) and Polyamide (PA) are frequently utilized in membrane fabrication as a base polymer ( Hamid et al., 2011; Nghiem, 2013; Faneer, Rohani, & Mohammad, 2016; Fujioka). Because of its great thermal stability and mechanical strength, PES is a promising polymer for membrane modification. However, lack of hydrophilicity causes fouling and low permeability considered a main failure of the PES membrane. Thus, recently some researches on incorporation of GO with PES membranes were applied to overcome these obstacles of using a PES as a base polymer (Wu, Tang, & Wu, 2014b; Mahmoudi et al., 2015). Therefore, this work aimed to fabricate PES and PES/GO 1 % membranes to improve membrane performance with enhanced water flux rate and better hydrophilicity which minimize the operation cost.
Figure 1:
Membrane processes classification according to the pore size
2. Materials and methods
2.1. Materials
Polyethersulfone (PES) granules were used from Goodfellow
Cambridge Ltd., England. The solvent 1-methyl-2-pyrrolidinone (NMP, 99.5%
purity) was of analytical grade and purchased from Merck Co., Germany.
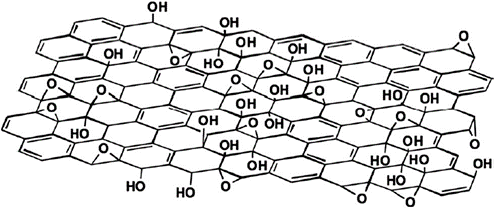
Ultrapure water was used as non-solvent. GO nano-sheets was synthesized in the
lab (refer to (Mahmoudi et al., 2015; Ba-Abbad, Mohammad, Mahmoudi, Faneer,
& Benamor, 2016). The chemical structure of graphene oxide is illustrated
in Figure 2.
2.2. Synthesis
of the Membranes
Nanocomposite membranes were created utilizing phase
inversion induced by immersion precipitation. For membrane fabrication, a
suitable amounts of PES (18 wt. %) were dissolved in solvent NMP (82 wt. %) with
mechanical stirring speed at 250 rpm for 6 h. Afterward, the prepared GO
nanoparticles with 1 % concentration were added into the mentioned polymeric
solutions. Mixing was followed by sonication for 30 min for breaking up
aggregates between nanoparticles. To thoroughly remove the air bubbles, the
produced solutions were left at room temperature for around 24 hours without
stirring. Then, using a film applicator, they were cast onto clean glass plates
with a consistent thickness of 200 m. Next, immerse the glass plate
horizontally in distilled water having an ambient temperature. The membranes
were maintained in fresh distilled water for 24 hours after primary phase
separation and solidification to guarantee complete solvent exchange. The
membranes could then be used. The polymeric solution compositions are presented
in Table 1.
|
Main Polymer |
Solvent |
GO amount |
Remarks |
|
PES
18 % |
NMP
82 % |
None |
|
|
PES
18 % |
NMP
82 % |
1
% |
Sonication
for 30 min |
Membrane
performance
The fabricated membranes performance was evaluated
through a dead-end stirred cell setup. The experiment was performed at fixed
pressure (4 bar). The pure water flux was calculated according to the following
equation
where Jw is the water flux (L/m2.h), V is the
permeate volume (L), A is the membrane effective area (m2), t is the
permeation time (h).
Contact
angle measurement
Using a Rame-Hart model 200 standard contact angle
goniometer with DROPimage Standard Software, the hydrophilicity of the
manufactured membranes was determined with an accuracy of 60.100.
The membranes were dried for 48 h and the medium used to measure the contact
angle was deionized water and air at ambient temperature (25 – 28 0C).
Field
emission scanning electron microscopy (FESEM)
FESEM Merlin Compact (Zeiss, Germany) was used to examine
the cross sectional structures of the fabricated membranes. Prior the FESEM
analysis, membrane samples were fractured into an appropriate size by using
Nitrogen liquid and mounted on the sample holder.
3. Results and discussion
3.1. Membrane
performance of water flux and hydrophilicity
Changes in the water flux of the membranes as a result of
blending of hydrophilic materials (GO) were investigated via the pure water
flux measurements. The GO-PES membrane shows higher water flux at 16 L/m2.h
comparing to PES membrane at 13 L/m2.h as presented in Figure 3.
To evaluate the hydrophilicity of blank and blended PES
membranes, the contact angle analysis was used. Figure 3 exhibits the results
of contact angle measurements for 1 wt.% of GO. The contact angle of blank PES
membrane decreases from 80.0º to 64.7º by blending with 1 wt.% GO. The
significant number of oxygenated groups of the GO nanosheets scattered in the
polymer matrix can be attributed to the improved hydrophilicity of GO/PES
membranes compared to blank PES. (Safarpour, Khataee, & Vatanpour, 2015),
this may have a positive impact on the blended membranes' pure water flux.
It clearly observed that the improvement in membrane hydrophilicity has the same trend of water flux increase. Water permeability is increased by enhancing the membrane's hydrophilicity, which attracts water molecules inside the membrane matrix and facilitates their penetration across the membrane. As a result of the high water flux, low contact angle, and good hydrophilic character of the produced GO-PES membrane, the results demonstrated that it performed well. Therefore, adding GO to PES membrane enhancing the membrane antifouling properties.
3.2. FESEM
The cross-sectional FESEM images of the prepared mixed
matrix membranes are presented in Figure 4.
The PES and GO-PES membranes have an asymmetric structure with a finger-like porous sub-layer and a skin top-layer. The addition of GO to the polymer matrix modified the membranes' finger-like shape slightly. Also, with the addition of GO to the membrane structure, the pore size of the membrane structure grows greater. The same finding was established by (Wu, Tang, & Wu, 2014a; Shanmugam, 2022) where the membrane pores became bigger as SiO2/GO added to membrane matrix.
a b
Figure 4: FESEM cross sectional images of (a) PES and (b) GO-PES membranes
4. Conclusion
GO nanocomposites are promising for high membrane
performance to obtain sustainable clean water. In addition, from the aspects of
nature of GO hydrophilicity, the membrane morphology and antifouling properties
provided efficient outcomes. As a result, it could be concluded that
incorporating GO NPs into PES membranes was an advanced method for developing
superior membranes with increased hydrophilicity and fouling control that were
ideal for a variety of purification and environmental applications.
References
Alammar, A., Park, S.-H.,
Williams, C. J., Derby, B., & Szekely, G. (2020). Oil-in-water separation
with graphene-based nanocomposite membranes for produced water treatment. Journal of Membrane Science, 603,
118007.
Ba-Abbad, M. M., Mohammad,
A. W., Mahmoudi, E., Faneer, K., & Benamor, A. (2016). Novel graphene-zinc iron oxide composite to enhance ultrafiltration
membrane performance for water treatment and desalination. Paper presented
at the Qatar Foundation Annual Research Conference Proceedings.
Baig, N., Sajid, M., &
Saleh, T. A. (2019). Graphene-based adsorbents for the removal of toxic organic
pollutants: A review. Journal of Environmental
Management, 244, 370-382.
Bolto, B., Zhang, J., Wu,
X., & Xie, Z. (2020). A Review on Current Development of Membranes for Oil
Removal from Wastewaters. Membranes, 10(4),
65.
Chung, Y. T., Mahmoudi, E.,
Mohammad, A. W., Benamor, A., Johnson, D., & Hilal, N. (2017). Development
of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced
antifouling and antibacterial control. Desalination,
402, 123-132.
Faneer, K. A., Rohani, R.,
& Mohammad, A. W. (2016). Polyethersulfone Nanofiltration Membrane
Incorporated With Silicon Dioxide Prepared by Phase Inversion Method for
Xylitol Purification. Polymers and
Polymer Composites, 24(9), 803-808.
Fujioka, T., & Nghiem,
L. D. (2013). Modification of a polyamide reverse osmosis membrane by heat
treatment for enhanced fouling resistance. Water
Science and Technology: Water Supply, 13(6), 1553-1559.
Hamid, N., Ismail, A. F.,
Matsuura, T., Zularisam, A., Lau, W. J., Yuliwati, E., & Abdullah, M. S.
(2011). Morphological and separation performance study of polysulfone/titanium
dioxide (PSF/TiO 2) ultrafiltration membranes for humic acid removal. Desalination, 273(1), 85-92.
Ismail, A. F., Khulbe, K.
C., & Matsuura, T. (2015). Gas separation membranes. Switz. Springer, 10, 978-973.
Kumar, P., Sharma, N.,
Ranjan, R., Kumar, S., Bhat, Z., & Jeong, D. K. (2013). Perspective of
membrane technology in dairy industry: A review. Asian-Australasian Journal of Animal Sciences, 26(9), 1347.
Mahmoudi, E., Ng, L. Y.,
Ba-Abbad, M. M., & Mohammad, A. W. (2015). Novel nanohybrid polysulfone
membrane embedded with silver nanoparticles on graphene oxide nanoplates. Chemical Engineering Journal, 277, 1-10.
Rao, G. P., Lu, C., &
Su, F. (2007). Sorption of divalent metal ions from aqueous solution by carbon
nanotubes: a review. Separation and
Purification Technology, 58(1), 224-231.
Rao, R. A. K., Singh, S.,
Singh, B. R., Khan, W., & Naqvi, A. (2014). Synthesis and characterization
of surface modified graphene–zirconium oxide nanocomposite and its possible use
for the removal of chlorophenol from aqueous solution. Journal of Environmental Chemical Engineering, 2(1), 199-210.
Safarpour, M., Khataee, A.,
& Vatanpour, V. (2015). Effect of reduced graphene oxide/TiO2 nanocomposite
with different molar ratios on the performance of PVDF ultrafiltration
membranes. Separation and Purification
Technology, 140, 32-42.
Shanmugam, K. (2022). Spray
coated cellulose nanofiber laminates on the paper to enhance its barrier and
mechanical properties. Journal of
Sustainability and Environmental Management, 1(1), 10-17.
Shannon, M. A., Bohn, P.
W., Elimelech, M., Georgiadis, J. G., Marinas, B. J., & Mayes, A. M.
(2010). Science and technology for water purification in the coming decades. Nanoscience and technology: A collection of
reviews from nature Journals, 337-346.
Teow, Y., & Solihuddin,
M. (2020). Process synthesis and optimization of SiO2/PVDF mixed-matrix
membrane for oil-water emulsion separation. Paper presented at the IOP Conference Series: Earth and
Environmental Science.
Wei, J., Vo, T., &
Inam, F. (2015). Epoxy/graphene nanocomposites–processing and properties: a
review. RSC Advances, 5(90),
73510-73524.
Wu, H., Tang, B., & Wu,
P. (2014a). Development of novel SiO2–GO nanohybrid/polysulfone membrane with
enhanced performance. Journal of Membrane
Science, 451, 94-102.
Wu, H., Tang, B., & Wu,
P. (2014b). Development of novel SiO 2–GO nanohybrid/polysulfone membrane with
enhanced performance. Journal of Membrane
Science, 451, 94-102.
Yeh, T.-F., Cihlář, J.,
Chang, C.-Y., Cheng, C., & Teng, H. (2013). Roles of graphene oxide in
photocatalytic water splitting. Materials
Today, 16(3), 78-84.
Zhang, R., Liu, Y., He, M.,
Su, Y., Zhao, X., Elimelech, M., & Jiang, Z. (2016). Antifouling membranes
for sustainable water purification: strategies and mechanisms. Chemical Society Reviews, 45(21),
5888-5924.
Zhu, X., Loo, H.-E., & Bai, R. (2013). A novel membrane showing both hydrophilic and oleophobic surface properties and its non-fouling performances for potential water treatment applications. Journal of Membrane Science, 436, 47-56.
|
|
©
The Author(s)
2022. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Related Article: Spray Coated Cellulose Nanofiber Laminates on the Paper to Enhance its Barrier and Mechanical Properties |